Hormones In Historical Perspective and The Future of Gender-Affirming Care
Hormones at the beginning of human history. Monarchy and the roles of eunuchs. The discovery and isolation of hormones. The first administration of hormones. The efficacy of cross-sex treatments.
This is going to be a somewhat exhaustive look into a very complex subject. The overall goal is for you to become an expert on the entire history and science of hormones. At the very end there should be very few people in the country who have a better grasp of the topic than you, and you should be able to confidently brag about your domain expertise to other people on Twitter or real life.
This isn’t about making a political stance, it’s just about gaining a high-level perspective on something important. So let’s dive in.
New therapies
About a year and a half ago was the first time I saw the phrase “gender affirming care” used on Twitter and since then the phrase has gained significant currency basically everywhere, I now see it mentioned in legislative proposals and policy agendas across state-lines.
The phrase is almost always used to refer to “hormone replacement therapy,” or the administration of cross-sex hormones to individuals experiencing gender dysphoria (the feeling of having been born the wrong sex) in an effort to treat some of the distressful presentations of the disorder.
I’ve seen really massive claims made about this treatment, mostly that it’s life-saving, but the people who say this almost never offer any evidence in support of their position, which is weird. But the people on the other side of the debate, the people who think gender affirming care is a synonym for serious harm, also offer zero support when they advance their ideas in the other direction, which is also weird.
If you think a course of action is really good, so good that it saves lives, or really bad, so bad that it puts lives in jeopardy, you’d think you’d want everyone to see as much evidence to substantiate your beliefs you can find.
Friend/enemy distinction
The big part of the problem is that politics is not a market for finding the best beliefs, it’s just a machine that helps you quickly identify who agrees or disagrees with your prior assumptions about the world, a conveyor belt that segregates who your friends and enemies are, to paraphrase Carl Schmitt. Any political idea roughly adjacent to what you know your natural community believes in will be agreed on reflexively, and the converse is true for political ideas not adjacent to your community’s broad ethos—its sentiments, tastes, values, etc.
This saves everyone a lot of time and very rarely gets anyone placed in the company of people they wouldn’t generally like. It’s a good, efficient way to maintain your circle of friends and keep the flow of ideas into your community relatively consistent.
But, on the other hand, it is a bad way to shape policy since sometimes our assumptions about the world are wrong, and we can end up getting results from a political program we believed in blindly that we wouldn’t have wanted if we just thought it all through.
An example that I think of every now and then is from the excellent period drama Downton Abbey, which I recommend if you haven’t seen it.
Downton as intro into Schmitt
The show takes place on an English estate called Downton in the early 20th century, at the cusp of a new Europe which saw the old landed gentry burn brightly before its final twilight brought on by war and modernization.
The estate houses the aristocratic Crawley family and a large retinue of servants—cooks, footmen, ladysmaids, valets, etc. One of the servants, Tom Branson, a walking irony, is an Irishman with strong antagonisms against the English crown, a socialist, and the Crawley’s chauffeur.
In one of the first scenes of dialogue with Tom, he’s driving Sybil Crawley, the daughter of Downtown’s lord, Robert Crawley, the Earl of Grantham, chatting with her about women’s suffrage. Tom remarks to Sybil that he believes her father is a representative of an oppressive class, the aristocracy, and that, more than freedom for Ireland or women’s rights, if he were a politician he’d want to see the yawning gulf between the rich and poor shut its unjust jowls.
During the course of the show’s events the Bolshevik revolution takes place in the Russian empire, and naturally Tom supports the Bolsheviks unthinkingly because of their adjacency to the cause of socialism.
When the Bolsheviks imprisoned the royal family of Russia in their own estate, Tom thought it was all an exercise in equality, which would have a peaceful resolution. He did not expect the innocent children of the Russian czar to be executed along with him.
Sometimes the efficiency that politics grants people looking to quickly find their friends and target their enemies ends up with people dying.
Humans are efficiency maximizers
This is probably partly why no one has offered evidence for or against gender affirming care, evidence isn’t the point, announcing to your community which side you’re on is the point. The other, smaller reason might just be that no one really knows anything about hormones beyond a few strong, colloquial beliefs.
The very notion of hormones almost has a superstitious quality in the collective American consciousness—hormones are a kind of explanatory bludgeon for a wide range of bodily events. Acne, weight gain, baldness, muscle growth, arousal, as well as any weird sensation, feeling, mental state, sudden illness and more can be chalked up to the involvement of hormones. I think most people feel confident that their intuitions about hormones are complete and feel like there’s no reason to even muster evidence when arguments about them come up.
But this still leaves the question of whose intuitions are right unanswered. Is gender affirming care good or not? Harmful or helpful?
Chasing the hormone question
So we’re going to start our investigation into the subject with a broad historical look at the earliest general understanding of the existence of hormones and their effects on the body and behavior, then look at how and when they were first isolated, and finally look at the first time we administered sex hormones in humans. From there we’ll look into the administration of cross-sex hormones and see what the scientific literature has to say about the matter.
When did humans first start messing around with hormones and why?
One of the strangest mysteries in the world is called the Sapients Paradox, or the question of why civilization only started around 11,000 years ago when anatomically modern humans, humans who are smart enough to build computers and planes and rockets, have been around for at least 100,000 years.
It’s a very weird problem, but it puts our starting point into an interesting perspective that I think is worth a digression: if this timeline is correct, then we are very late to the game as a species and probably still have a lot to learn.
One prevailing feeling these days is that humanity has reached the apex of all understanding, that mysteries are a thing of the past. And I understand this. We can fly through the air faster than sound can travel, speak into magic mirrors and invoke our loved ones wherever they are, see through flesh without parting the body from its integrity, and we even have near infinite food and water that we can summon at will.
It feels like we’ve arrived at the end of knowledge. But it’s more than likely that we’re only just at the beginning. It took us 90,000 years just to start farming, we may be 90,000 years out from another big leap of growth.
Post-Dawn
Around 3,000 years after the dawn of civilization (so 8,000 years ago), It’s speculated that various human groups independently discovered that castration of male livestock made them much more useful and easier to manage as draft animals, and the practice became widespread.
Castration prevents unwanted breeding of low quality livestock, pacifies of otherwise aggressive bulls used for plowing and other work, and improves meat quality in livestock bred for consumption. The flavor of beef mostly comes from its fat content, and castration increases fat deposition as well as meat tenderness in cattle.
Living in an advanced civilization means that most of us are disconnected from our food sources these days and have little if any idea of how animals are transformed into our dinner, so it was interesting to learn that beef is mostly tender and tasty because of this practice that we discovered thousands of years ago.
Rumination
As a side note, during my research here I stopped and wondered: we humans grow throughout our lives and maintain our bodies with the help of the animal proteins we eat, so how is it that cows and other ruminants are so large, much larger than we are, when they never eat other animals?
Ruminants get their name from a stomach compartment called the rumen, the largest of two smaller stomach compartments they possess in addition to a “true stomach” called the abomasum.
Metabolism in ruminants starts similarly to the way it begins in humans, with mechanical (chewing) and chemical breakdown of food turning large molecules into smaller ones more readily usable by cells, but the first big difference is found in the kind of food being consumed, with the ruminant diet being made up entirely of plants.
Plants are comprised mostly of carbohydrates, as well as a small amount of proteins and lipids. The carbs are broken down by extracellular enzymes into simple sugars, fermented, and then broken down further by rumen bacteria and protozoa into three important compounds—butyrate, propionate, and acetate—which are known as Volatile Fatty Acids (VFAs). These VFAs enter the bloodstream of the ruminant through the rumen walls, and make their way to the liver to be used as energy for things like milk production, pregnancy, and general physical maintenance:
Ruminants also break down the small amount of proteins in plants into amino acids needed for protein synthesis, but additionally synthesize their own proteins from the breakdown of a byproduct of protein synthesis called urea, which is then turned into ammonia. Ammonia, which can also be derived from other sources besides urea, is broken down into glutamic acid or glutamine, an amino acid, which is then used by microorganisms in the rumen as a major component of their own protein production. Ammonia can also be broken down into a number of other amino acids.
It’s a very complicated process, but having a diet low in protein means that ruminants depend in large part on the endogenous proteins they produce in their own gut, which is pretty cool. It’s also funny that “ruminating” doesn’t just mean “thinking about something,” the term comes from ruminant animals and describes the process of “chewing the cud,” or chewing regurgitated plant matter for further breakdown in the rumen. This back and forth between the mouth and the rumen apparently happens between 50-70 times before plant matter is fully digested in cattle.
When was castration first used to modify human behavior?
We’ve known for a long time how to use the hormonal changes of castration to our advantage in animal agriculture, but when did we start applying the same ideas to humans?
Here’s a history snippet from the American Urological Association’s Journal of Urology, titled From Torture to Therapy: The History of Human Castration:
“The earliest records for intentional human castration to produce eunuchs are from the Sumerian city of Lagash in the 21st century [BCE]. Ancient Greeks and ancient Romans used it to produce eunuch slaves. During Byzantine Empire, it was considered such as a punishment. In Arabian countries castration started to be used to obtain faithful harem guardians since 750 [CE] and then, during the Ottoman Empire, the Imperial Harem was a powerful institution of the Sultan. From the late 16th century castration was carried out in Italy to preserve the unbroken male voice into adult life.”
Monarchy is likely the most archaic form of formal government, with the oldest records of the institution dating back to around 5,100 years ago in Egypt with the rule of Pharaoh Narmer in 3100 BCE, and about 4,600 years ago in Sumer with the rule of Enmebaragesi in 2600 BCE.
As far back as 4,000 years ago civilizations are recorded to have used castration on humans as a form of punishment, but also as a functional tool to circumvent some of the emergent problems of monarchy, with the biggest ones being increasingly autonomous vassals and issues of succession. Power-hungry courtiers seeking to extend the reach of their rank and establish their own dynasties and royal sons feuding for the throne of their father have been frequent nexuses of monarchical strife.
The Old Kingdom
One of the oldest examples of emboldened vassals of a royal court causing trouble for a kingdom is the fall of the Old Kingdom of Egypt, or the "Age of the Pyramid Builders,” around 2200 BCE. The middle period of the Old Kingdom, c. 2500 century BCE, saw the unprecedented decentralization of pharaonic authority following a series of reforms by the Old Kingdom pharaoh Djedkare Isesi, who allowed a significant transferral of power to provincial rulers called nomarchs, the administrators of Ancient Egypt’s territorial divisions, or nomes.
The position of the normachs became hereditary not long after Djedkare’s reforms, incentivizing the accumulation of power and resources over time and allowing these local governors to become increasingly independent from Memphis, the center of Ancient Egyptian authority. One of the last pharaohs of the Old Kingdom, Pepi II, allowed further accrual of resources by the nomarchs, who raised their own armies, built their own tombes, and eventually entered into conflicts with neighboring provincial regions:
“Increasing wealth and power appears to have been handed over to high officials during Pepi II's reign. Large and expensive tombs appear at many of the major nomes of Egypt, built for the reigning nomarchs, the priestly class and other administrators. Nomarchs were traditionally free from taxation and their positions became hereditary. Their increasing wealth and independence led to a corresponding shift in power away from the central royal court to the regional nomarchs.
Later in his reign, it is known that Pepi divided the role of vizier so that there were two viziers: one for Upper Egypt and one for Lower, a further decentralization of power away from the royal capital of Memphis.”
The pharaohs succeeding Pepi II were promptly overthrown by the ambitious, power hungry nomarchs of Heracleopolis Magna, the capital of a nome in ancient Upper Egypt, leading to an era of strife called the First Intermediate Period. The period saw the ambition of neighboring nomarchs turn into mutual warfare and attempts to expand their territories, as with the best known warlord and provincial governor from the era, Ankhtifi, conquering nomes to the south of Heracleopolis and attempting to then expand to Thebes in the north.
Principal-Agent Problems and Eunuchs
The principal-agent problem is an economic idea describing the natural conflict of priorities which arises between an owner of an enterprise and the individual delegated to execute its functions. Different people are different, they can’t all have the same vision or ideas or goals, and an oceans’ worth of ink has been spilled on the subject of “management styles” and “management solutions” to try and help businesses align their members with the priorities of their founders. The principal-agent problem is such a huge hurdle to the success of any collaborative effort that the most impressive thing about any successful enterprise is probably that they’ve somehow gotten more than two people in a room to agree on one thing at a time.
Civilizations of old resolved the principal-agent problem, a problem inherent to the first forms of formal government, by using early knowledge of hormones to disrupt puberty in order to create an entirely new kind of person. In the same way that animal castration creates something more manageable, ancient societies found the same to be true in men. They used this knowledge to make a much meeker, more quiescent human: The eunuch, a man with zero ability to form relationships, produce heirs, accumulate wealth and resources over time, but who also had significantly altered behavior which tended toward a pacified loyalty, which we’ll get to a bit later.
Here’s a good explainer from an old paper titled, The Political Functions of Eunuchism, which succinctly lays out the importance of castration in the maintenance of royal power:
“In the great oriental empires the head of the state mobilized the country's resources with the aid of a centralized bureaucracy which helped counteract centrifugal and feudal tendencies. Yet the bureaucratic forms of administration also involved the danger that the ruler would become only the primus inter pares among the top office holders, a servant of the apparatus rather than its master. Recourse to political eunuchism hence was one device by which the ruler assured himself of a body of personally loyal instruments who could be used against bureaucrats and feudal opponents alike. Rootless men could be used to great advantage wherever a ruler needed to defend himself against recalcitrance or opposition from men entrenched in rationalized bureaucratic positions, or in territorial and kinship groups.”
Physical characteristics of eunuchs and their roles
A sure-fire way to prevent your royal bureaucracy from attempting to take on greater levels of autonomy is to make that bureaucracy pacified through castration, which early humans learned created many different physical changes that allowed eunuchs to function in their various roles.
Here’s a great excerpt about one of the major traits of eunuchs—their very distinct, highly neotenous appearance. From a great research article titled Eunuchs In Historical Perspective:
“Childhood castration, castration before puberty, was done in hopes of creating an individual who was significantly different from a normal man.
Boys castrated before puberty developed a distinctive appearance and perhaps a distinctive personality. Because they never completed normal puberty and missed the developmental changes brought about by the adolescent phase of testosterone production, men castrated as children remained beardless with fresh, clear complexions and had patterns of fat deposition characteristic of women. The epiphysial plates, that is the growth plates, in their long bones did not close at puberty, resulting in an individual with unusually long arms and legs and a tall, though frail stature. The bones of the lower face did not mature, resulting in a triangular face with a small chin. Their hair was thick and luxuriant and did not fall out as they aged. Their voices remained high pitched, and their musical ability was admired and cultivated in Europe from the fourth into the nineteenth centuries. While these eunuchoid characteristics are not necessarily aesthetically favored today, they have been appreciated in some cultures in the past. Prepubescent castrates acted as courtiers in Hellenistic, Byzantine, Muslim, and Chinese courts.”
The ancients probably had some ideas about disease causation
The article goes on to state that testosterone deprivation suffered by court eunuchs led to common health problems like accelerated aging, osteoporosis, various cardiovascular diseases, and diabetes, and reading all of this, It’s hard not to wonder what physicians in antiquity thought about these disease trends. I think us moderns assume that establishing a causal link like the one between a hormone-producing organ and certain Illnesses would have been beyond the ancients’ abilities, but it’s surprising to learn how much they actually knew, and worth considering that they probably had a good deal of understanding about how our organs contribute to the health of the body.
The most interesting example of how comprehensive ancient knowledge actually was is probably Aristotle’s theory of biology, which I think would surprise most people to know that the 4th century BCE philosopher developed. Aristotle’s biological writings made up almost a third of his entire body of work, and his methods of inquiry almost exactly resembled those of modern biologists in the field, centering around empirical observation, data gathering, and drawing inferences from general patterns, with some considering him the founder of the science of biology. Aristotle’s works describe essential biological processes from metabolism, temperature control, sensory processing, embryo development, to genetic inheritance, which is just wild. His observations would go on to be influential across multiple civilizations for thousands of years.
The ancients probably had more understanding of the body and its processes than we give them credit for.
Roles of eunuchs
Across historical periods and cultures the same tasks in royal courts have tended to be reserved for eunuchs, with these occupational parallels seemingly arising out of the near universal meme that celibacy and sanctity are interlinked. In Byzantium, ancient China, and the Islamic empires, eunuchs served as sentries who guarded the unassailable chambers of the king and as servants who attended him beyond this lofty threshold, serving him food and clothing him, and acted as protectors of the king’s royal accouterments, harem, and executors of court ceremonies. Domains considered solemn and sensitive to profination were thought to be preserved by putting them in the care of a eunuch.
The Castrati
Eunuchs have been teachers, court physicians, managers of royal treasuries and properties, and even centerpieces of aristocratic entertainment, with one of the most interesting examples being the castrati, a class of prepubescent boys castrated to preserve the higher-pitched voice of youth and create a unique and highly coveted sound, the castrato, the male-equivalent of a soprano.
The castrati dominated the European opera scene for centuries, with their use spanning from the 16th to around the 19th centuries CE, and with many achieving levels of renown equivalent to modern day pop icons. The training of castro youths in Italian operas was wildly rigorous:
“The regimen of one singing school in Rome (c. 1700) consisted of one hour of singing difficult and awkward pieces, one hour practising trills, one hour practising ornamented passaggi, one hour of singing exercises in their teacher's presence and in front of a mirror so as to avoid unnecessary movement of the body or facial grimaces, and one hour of literary study; all this, moreover, before lunch. After, half an hour would be devoted to musical theory, another to writing counterpoint, an hour copying down the same from dictation, and another hour of literary study. During the remainder of the day, the young castrati had to find time to practice their harpsichord playing, and to compose vocal music, either sacred or secular depending on their inclination.”
Androgen deprivation and behavior
The best word to describe the qualities of eunuchs is probably “agreeable,” as the word is used in the context of Big Five personality traits, the most prominent taxonomy for describing individual character in behavior science. The other four traits are extroversion, neuroticism, conscientiousness, and openness to experience, and the general idea of this taxonomy is that these five superordinate traits describe near-exhaustive range of subordinate behaviors. Depressed, anxious, passive-aggressive, and impulsive behaviors being subsumed under the neuroticism category, for example.
Eunuchs were assumed to be compliant, and modern data on the effects of androgen deprivation (which happens when you’re either castrated physically, chemically, or born with certain genetic disorders) seems to point to this being the case.
Testosterone’s role in the Big Five trait of extraversion
To start off connecting androgen deprivation to compliance, let’s see what the role of androgens are in a healthy adult, Here’s a good breakdown on the role of the most prominent androgen in men, testosterone:
“Testosterone has been found to be most reliably associated with extraversion-related traits, particularly characteristics related to social dominance. Traits such
as dominance, aggression, assertiveness, and status-seeking are conceptualized as further facets of social dominance, and have been studied in both humans and other animals—with many finding associations between these behaviors and testosterone. For example, in human status seeking, individuals with higher basal testosterone levels seek out higher social standing; those with lower testosterone prefer (or are relegated) to stay in lower social positions rather than ascend hierarchies.”
Androgen deprived individuals are slightly more agreeable
More research needs to be done on the small, understudied cohort of androgen deprived males, but the limited findings in the area have confirmed some association between androgen deprivation and agreeableness, with the concluding paragraph of this paper titled Castration and personality, saying:
“Our data reveal that the BFI [Big Five Inventory] personality trait of agreeability is elevated in androgen-deprived males compared to eugonadal controls. This shift becomes more pronounced and statistically significant with the addition of supplementary estrogen following androgen-deprivation. An increase in agreeability likely made androgen-deprived males more suitable for the senior administrative, diplomatic, and military positions they historically held.”
Which confirms what we suspected. But what is a hormone anyway? And what do sex hormones do in the body?
The what, how, and why of sex hormones
A hormone is a cellular signal involved in metabolism, growth, sexual function, cognitive function, and more, with the major hormone-producers in the body being the endocrine glands, which includes the pituitary, pineal, thymus, and hypothalamus, all located in the brain, the thyroid and parathyroid, located in the neck, the adrenals located on both kidneys where they produce things like adrenaline and cortisol, the “stress hormones,” the pancreas, where insulin is made to regulate blood sugar, and the gonads where things like testosterone and estrogen are produced.
There are only two kinds of glands in the body, endocrine and exocrine. Endocrine glands secrete compounds directly into the bloodstream, exocrine glands like sweat, mammary, and salivary glands secrete substances through openings on the body.
Protein and steroid hormones
The hormones made by the sex organs and adrenal glands are the only non-protein derived hormones in the body, with all other hormones being made or derived from the synthesis of proteins from amino acids. When you eat protein you aren’t just building muscles, you’re building a wide array of hormones too.
The sex hormones and the hormones made by the adrenal glands are steroids, a kind of hormone derived from the conversion of cholesterol—which is synthesized in the liver from the breakdown of glucose into a molecule called Acetyl-CoA—into pregnenolone, and the conversion of pregnenolone into various hormone products. We hear so often that cholesterol is bad, but not nearly enough about how it’s the sole precursor of things like testosterone and estrogen. And it’s worth mentioning that high levels of dietary cholesterol probably aren’t associated with any of the disease risks that we’ve heard repeated at length over the last several decades.
From a systematic review of the literature on the association between cholesterol and cardiovascular disease:
“Dietary cholesterol has been suggested to increase the risk of cardiovascular disease (CVD), which has led to US recommendations to reduce cholesterol intake. The authors examine the effects of dietary cholesterol on CVD risk in healthy adults by using systematic review and meta-analysis…Forty studies (17 cohorts in 19 publications with 361,923 subjects and 19 trials in 21 publications with 632 subjects) published between 1979 and 2013 were eligible for review. Dietary cholesterol was not statistically significantly associated with any coronary artery disease (4 cohorts; no summary RR), ischemic stroke (4 cohorts; summary RR: 1.13; 95% CI: 0.99, 1.28), or hemorrhagic stroke (3 cohorts; summary RR: 1.09; 95% CI: 0.79, 1.50).”
Gonadotropins
Gonadotropins, glycoprotein hormones secreted by the anterior lobe of the pituitary gland, play major roles in sexual function and influence sex hormone production, though they aren’t considered sex hormones. The major gonadotropins are luteinizing hormone, which stimulates ovulation in women and testosterone production in men, follicle-stimulating hormone, which stimulates the growth and development of sex cells in both men and women, and gonadotropin-releasing hormone, which controls the release of both of the preceding hormones.
Getting into the sex steroids
The main sex hormones in the body are the androgens, the estrogens, and the progestogens, with all three sex steroids being produced in both men and women in varying amounts, and each serving various sex-specific functions in growth and development, which we’ll get into.
The androgens
Here’s a good, short overview of what androgens are:
“An androgen, or male sex hormone, is defined as a substance capable of developing and maintaining masculine characteristics in reproductive tissues (notably the genital tract, secondary sexual characteristics, and fertility) and contributing to the anabolic status of somatic tissues. Testosterone together with its potent metabolite, dihydrotestosterone (DHT), are the principal androgens in the circulation of mature male mammals.”
Androgens are considered male hormones because men produce over ten times the amount of the steroid than women, and they are known to cause characteristically masculine changes in women who were either exposed to higher levels of androgens in utero because of genetic or other disorders, who develop excess androgen production because of conditions like polycystic ovarian syndrome, or who are administered exogenous male sex steroids in adulthood.
We’re going to focus a lot on this hormone because it’s both a direct player in the physiology of both sexes, but also a major precursor of estradiol, the most common estrogen in men and women which tightly regulates reproduction in the latter, and which also tightly governs metabolism and brain function in both groups.
DHT and testosterone
Dihydrotestosterone (DHT) is the most potent androgen in men (meaning it occurs in smaller amounts than testosterone but has greater affinity with androgen receptor sites) and is produced by a reaction between testosterone and an enzyme called 5α-reductase. DHT is involved in some of the most important stages of male life, contributing to the early formation of male sexual features during embryo development, some of the masculinizing changes of puberty like body hair growth, and eventually the development of the signs of aging in men like male pattern baldness (my biggest fear, I shudder at the mention of it) and prostate diseases. DHT is mostly involved in the peripheral development of male secondary sex characteristics.
The less potent but more prevalent androgen testosterone is synthesized from cholesterol and secreted by Leydig cells in the testes (and in Theca cells in the ovaries), and plays a significant role in the development and maturation of both primary and secondary male sex characteristics and in anabolism, one of the two major aspects of metabolism (the other being catabolism, the breakdown of large molecules into smaller components usable by cells) which centers around the building up of cellular materials for use in the body. Testosterone’s anabolic effects include the promotion of growth in tissues with androgen receptors like bones and muscles, as we’ll see.
Testosterone’s involvement in development
DHT and testosterone contribute to sexual differentiation and the maturation of male sexual features in utero, and testosterone masculinizes the brain of developing male embryos leading to gender-typical behaviors and interests in early life as well as larger brain volume, while a protein in female fetuses called alpha-fetoprotein inhibits the masculinizing effects of testosterone on the female brain.
In puberty testosterone production is stimulated by the hypothalamic-pituitary-gonadal axis, a system of hormonal communication between the brain and reproductive organs. At the end of adolescence the hypothalamus begins releasing huge amounts of gonadotropin-releasing hormone, a chemical signal which stimulates the release of luteinizing hormone and follicle-stimulating hormone from the pituitary gland. Luteinizing hormone prompts the release of testosterone in the testes and ovaries, and finally the testosterone in men and testosterone converted into estrogens in women go on to induce the dramatic changes seen in puberty through a system called the growth hormone/insulin-like growth factor-1 (IGF-1) axis.
The body is complicated
The body is the most complicated thing in the world, a huge chunk of what we know about these complex systems has been discovered in the last few years. Phones, cars, planes, LLMs, they’re all impressive, but none come close to the body, every cell is a cathedral, every system is a well-arranged symphony. We take it for granted.
Growth Hormone/IGF-1 axis in puberty
During puberty in men testosterone has significant stimulatory effect on the pituitary, which releases growth hormone, an anabolic hormone important in chondrocyte (cartilage cell) proliferation and much else, which then stimulates the release of insulin-like growth factor-1 in the liver, a hormone with wide-ranging growth promoting effects on various tissues and which mediates and enhances the action of growth hormone. During female puberty estrogens act on the same hormonal axis but produce less a pronounced stimulatory effect, and less growth in turn.
In adolescence, long bones in the body like the femur, tibia and fibula, the humerus, radius, and ulna have sections of cartilage at their ends called epiphyseal plates, or “growth plates.” IGF-1, in response to increased testosterone production, is secreted at high levels during puberty, and the hormone (in conjunction with growth hormone) dramatically increases chondrocyte proliferation in the epiphyseal plates, lengthening them. The chondrocytes mature in the extracellular matrix (or ECM, the scaffolding which makes up tissues like cartilage) of the growth plates and become larger, eventually undergoing a process called endochondral ossification where they secrete tiny vesicles (extracellular organelles) that accumulate calcium and phosphate ions and form hydroxyapatite crystals, which then bind to the ECM of cartilage and cause calcification.
The calcified cartilage gets broken down by cells called osteoclasts, which are essential for maintaining the integrity, repair, and remodeling of bones. Bone forming cells called osteoblasts then begin depositing collagen and hydroxyapatite crystals into the calcified cartilage to form mature bone tissue. An interesting side note: This process is also how the body repairs severe fractures. When you break a bone, cartilage cells proliferate and fill the gap between broken segments of bone and eventually mineralize and harden the same way epiphyseal plates do in puberty.
IGF-1 and growth hormone also contribute to the increased size of the heart, lungs, liver, and other organs in puberty.
Testosterone in both men and women
In young men and women, testosterone causes growth of the penis and clitoris, and increases libido. While in men testosterone, through its stimulation of the growth hormone/IGF-1 axis, causes significant growth to the skeleton and development of larger shoulders and rib cages, and facial bone remodeling which includes the accentuation of the brow ridge, jaw, and chin. The voice also deepens, there’s growth of cartilage on the thyroid (the Adam’s apple), and muscle mass increases markedly. Testosterone mediated changes to the musculoskeletal system occur in women as well but are less pronounced because of testosterone’s more central role as a precursor to estrogens, which act on epiphyseal plates as maturation accelerants, bringing about the completion of puberty much more rapidly in women than in men.
Testosterone and sex segregation in sports
After puberty men produce around 15 times more testosterone than women, leading to significant differences in muscle mass, lung volumes, airway size, maximum expiratory flow (a measure of how well lungs expel air), cardiac output and oxygen saturation at peak exercise, and a lot more. The broad advantages men have over women along physical dimensions makes sex segregation in sports a necessary condition for fairness:
“Elite athletic competitions have separate male and female events due to men’s physical advantages in strength, speed, and endurance so that a protected female category with objective entry criteria is required. Prior to puberty, there is no sex difference in circulating testosterone concentrations or athletic performance, but from puberty onward a clear sex difference in athletic performance emerges as circulating testosterone concentrations rise in men because testes produce 30 times more testosterone than before puberty with circulating testosterone exceeding 15-fold that of women at any age.”
Testosterone in adulthood
In both male and female adulthood testosterone maintains bone and muscle mass through the process of aromatization, the conversion of testosterone into the regulatory hormone estradiol, with declines in testosterone being associated with osteoporosis and sarcopenia in older folks. Testosterone also influences fat distribution, mood, behavior, arousal, and sexual function in both sexes.
The estrogens
The main estrogen in both men and women is a hormone called estradiol, which is converted in tissues like bone from testosterone by the enzyme aromatase (an enzyme also highly prevalent in adipose tissue, so the more fat you have the more estrogens your body will make), and which women synthesize in the ovaries from androgens.
The average amount of estradiol in premenopausal women is between 30 to 400 pg/mL (picogram per milliliter, a picogram is one-trillionth of a gram), 0 to 30 pg/mL in post-menopausal women, and 10 to 50 pg/mL in men, meaning that women have between 3 to around 10 times more estradiol than men on average.
Other estrogens
Besides estradiol (also written as E2 in scientific literature), the dominant form of estrogen produced in the ovaries during women’s reproductive years, the two other common, but much less potent forms of estrogen are estrone (E1) and estriol (E3). Estrone becomes the most dominant form of estrogen in postmenopausal women, converted in tissues from androstenedione, a weak androgen produced by the adrenals. Estriol is produced in huge amounts by the placenta during pregnancy.
The role of estrone in postmenopausal health is less than clear, with some research finding that high levels of the estrogen being important for bone and intestinal health but other research showing a positive correlation between estrone and breast cancer risk. The role of estriol during pregnancy is also unclear, with the most important estrogen in pregnancy being estradiol, which plays a role in modulating the immune system in order to strike a balance between protecting the developing fetus from pathogens and ensuring the heightened immune system doesn’t attack the fetus and cause an unwanted termination. (The most common progestogen in the body, progesterone, has a much more potent immuno-modulatory and immune-enhancing role in pregnancy, as we’ll see in the next section).
The female immune system
Estradiol and progesterone occur in much more significant amounts in women over their lifetimes than men, conferring a significantly stronger immune system, but likely also contributing to the much higher incidence of auto-immune disorders (women are something like 2-8 times more likely to have an autoimmune disorder than men). “Women show more vigorous T and B cell responses and have higher circulating CD4 T cell numbers than men,” this review of progesterone says, meaning that women respond to pathogens with a much insane force and effort.
In every infectious disease epidemic I looked at, men always succumbed more often than women, likely because of the variance in hormone production. During the bubonic plague, men died more than women. During the Spanish flu, men died 50% more often than women. During measles outbreaks the world over, men died more often than women. During covid, men died much more often than women. Men are bigger, faster, and stronger, but the sex that carries the next generation into existence has extreme resistance to environmental threats.
Estrogens as regulatory hormones
While I was reading about the role of estrogens in the body, a picture started to emerge of their importance as a class of regulatory hormones, which is something I’ve never heard said before. Without estrogens, things go very wrong in the body, with things like bone growth going out of control. The best illustrations were clinical accounts of men who had genetic disorders that caused estrogen deficiency and estrogen insensitivity. Here’s an account I read of an individual with estrogen insensitivity, meaning that no matter how much estrogen his body produced, the hormone had no activity in cells and tissues:
“Strong support for important roles of E2 [estradiol, the most common estrogen in men and women] comes from studies in men with inactivating mutations of either ERα [estrogen receptor A, one of two places where estrogen binds to cells to produce responses] or aromatase [an enzyme in tissues that converts testosterone into estradiol]. Estradiol insensitivity was found in a 28-year-old man diagnosed with a homozygous ERα mutation that produced a truncated nonfunctional protein. The individual presented with continued linear growth and tall stature due in part to unfused epiphyses [growth plates], despite normal serum testosterone. Significant osteoporosis was noted, indicating that endogenous estrogen and ERα are important in men for normal bone growth and development. This individual was also overweight for his height and showed excess abdominal fat. Elevated endogenous estrogen levels in this individual failed to suppress the pituitary gonadotropins, luteinizing and follicle-stimulating hormones (LH and FSH, respectively) in the absence of the functional ERα receptor. Thus, while direct action of male sex steroids at ARs [androgen receptors] in the brain may play some role in negative feedback that regulates LH and FSH, estrogen signaling via ERα is also required.”
And here’s a clinical account of what mutations causing estrogen insensitivity do in women:
“Estrogen insensitivity syndrome is a very rare condition characterized by a defective ERα that is insensitive to estrogens. The clinical presentation of a female was observed to include absence of breast development and other female secondary sexual characteristics at puberty, hypoplastic uterus, primary amenorrhea, enlarged multicystic ovaries and associated lower abdominal pain, mild hyperandrogenism (manifested as cystic acne), and delayed bone maturation as well as an increased rate of bone turnover. . .
Genetic polymorphisms in the gene encoding the ERα have been associated with breast cancer in women, gynecomastia in men and dysmenorrhea.”
Estradiol as metabolic skeleton
Declines of estradiol, either as a result of menopause or age-related primary and secondary hypogonadism (testicular dysregulation and hypothalamic-pituitary axis dysregulation) in men, lead to lipid metabolism disorders like coronary artery disease, peripheral artery disease, and cerebrovascular disease, as well as metabolic syndrome, which is a superordinate disease classification including high blood pressure, hyperglycemia, diabetes, and obesity.
If testosterone is the building hormone, estrogens are like an engineer and architect, drawing out and maintaining the integrity of extremely complex projects.
Estrogens and the brain
Beyond being necessary in regulating the extraordinarily error-sensitive processes of pregnancy, immune function, and metabolism, one of the most interesting things about estrogens are their role in regulating the health of the brain. Age-related declines in memory and learning are closely associated with declines in estrogens, as is increased incidence of sleep disturbances, depression, schizophrenia, Alzheimer’s, Parkinson’s, and more through the wide-ranging role of estrogens in maintaining serotonergic, dopaminergic, and various other brain systems.
The progestogens
The main progestogen in men and women is called progesterone and is synthesized in the gonads from cholesterol. Outside the luteal phase of the menstrual cycle (the two week phase preceding menstruation and ovulation which includes PMS), men and women produce the same small amount of progesterone on average, about .2 to 1 ng/mL (nanogram per milliliter, a nanogram is a billionth of a gram). During the luteal phase women produce 2 to 25 times more progesterone than men to prepare the lining of the uterus for the implantation of a fertilized egg.
During pregnancy women produce between 100 to 600 ng/mL of progesterone, which is why the steroid is considered the pregnancy hormone. Declines in progesterone during pregnancy are more closely tied with miscarriage than any other sex steroid. During pregnancy progesterone inhibits the hypothalamic-pituitary-gonadal axis to prevent a continued menstrual cycle and subsequent end of gestation, the steroid also inhibits lactation until after birth while allowing the mammary glands to develop.
Progesterone and development
Progesterone also acts to globally develop the fetal central nervous system, being associated with the development of neural stem cells which go on to mature into distinct neurons like ones belonging in the cortex, which is the outer, convoluted layer of the brain responsible for higher-order functions like sensory perception, language acquisition, and thought, the hippocampal dentate gyrus, a brain region associated with memory formation, information apperception, and pattern recognition, and the dopaminergic system, which is responsible for reward-seeking and goal-orientation, along with much else.
Brain and central nervous system networks are made up of neurons, or nerve cells, which come in different forms depending on the region of the body, and which send and receive signals to one another and to other bodily systems to govern their function. Neurons communicate with each other in part through connecting tissue called axons, which carry electrical signals rapidly to and from other nerve cells. Axons are insulated by a kind of fat called myelin, myelin helps increase the transmission speed of axonal signals, and the loss of myelin causes a slew of central nervous system diseases like multiple sclerosis. Progesterone is massively important for the growth of myelin in early brain development by stimulating myelin progenitor cells called oligodendrocytes, and for maintaining the integrity of myelin throughout adulthood.
The steroid has been known to cause the restoration of lost myelin in the brains of pregnant women by stimulating increased oligodendrocyte proliferation.
Here’s a bit more on progesterone and early brain organization:
“Neurons and glial cells form complex functional networks implicated in several functions carried out by the CNS [central nervous system]. Although the arrangement of these networks, usually referred to as neural circuits, varies according to the related function of each CNS region (LoTurco, 2000), the processes that contribute to the formation of such neural circuits are similar throughout. The general mechanisms that shape the neural circuitry occur late in neurodevelopment and include neuronal maturation, axons and dendrites arborization, axon guidance supported by glial cells, the establishment of synaptic connections between neurons and the elimination of improper connections (Weiner et al., 2013). Progesterone contributes to the organization and the establishment of neural circuits during mammal development by promoting cell maturation, dendrites formation, synaptogenesis and axonal myelination, as documented in rats and guinea pigs (Sakamoto et al., 2001; Tsutsui, 2008; Palliser et al., 2012).”
More on progesterone and the brain
Here’s a bit more on the fact that pregnant women and fetuses are known for being extremely resilient to brain injuries, likely because of this steroid:
“Animal studies have shown neuroprotective effects for progesterone, which protects, for example, against necrotic damage and behavioral abnormalities caused by traumatic brain injury, e.g. by increasing the activity of antioxidative catalase [catalase is an enzyme that converts harmful intracellular hydrogen peroxide into harmless water and oxygen] or by modifying the microtubule-associated protein-2 [MAP2 helps stabilize brain networks] content. In this context, progesterone and allopregnanolone [a progesterone byproduct] inhibited cell death and cognitive deficits, including recovery of select behaviors after a contusion of the rat prefrontal cortex. Progesterone-mediated neuroprotection has also been reported in peripheral nerve and spinal cord injury.”
Progesterone is also associated with protection against certain types of epilepsy, depression, and panic disorders. Let’s be honest, God really went off when He invented this hormone, you have to give Him credit.
Progesterone in men
Beyond the importance of the steroid in the development of the brain and nervous system in early life, little is known about the importance of progesterone in adult men. There is some research suggesting that progesterone is involved in the kidneys, liver, lungs, and reproductive organs, but more investigation needs to be done here to establish the full importance of progesterone in men.
An interesting thing to note is that when men are given exogenous progesterone, nothing really significant happens as it does when they’re given estradiol. Exogenous estradiol feminizes men in a few ways, promoting breast growth, heightened voice pitch, etc., but administering progesterone just seems to make men kind of sleepy. Since most of the studies I read had extremely small samples, these findings don’t mean that progesterone does little in male physiology, it just means our understanding of the steroid is probably still pretty early.
Hormones in recent history
Sex steroids represent a complex but wildly important nexus in human knowledge; over the last several thousand years humans have successfully applied our rudimentary understanding of sex hormones to aid our efforts in agriculture and formal governance, and we’re going to see the fruition of this understanding in the discovery and administration of sex steroids in the early 20th century, which eventually led to debates on the use of cross-sex hormones we’re having now.
We’re going to look at the earliest and most popular commercial hormone products to gain some insight into the health effects of hormones administered externally. If early same-sex hormone therapies were helpful or if they at least showed clinically insignificant results, we can assume that something similar could possibly be true of cross-sex hormone treatments. If same-sex hormone therapies show obviously negative outcome trends, that probably bodes poorly for exogenous hormone administration as a whole.
Either way we’ll take a look at some of the research around today’s common cross-sex hormone treatments near the end to hopefully settle the questions about their safety and efficacy for good.
The discovery of sex steroids
Adolf Friedrich Johann Butenandt, a German biochemist with the most German-sounding name in the visible universe, a Nobel prize winner and president of the Max Planck Society from 1960 to 1972, independently discovered the sex steroid estrone in 1929 at the same time as Edward Adelbert Doisy, an Illinois-born biochemist and Nobel prize winner who also happened to be the discoverer of vitamin K. Both men were aware of the significant presence of female sex hormones in the urine of pregnant women in the early 1920s, and both were eventually able to extract and purify estrone from pregnant women’s urine and lay the foundation for further developments, though only Butenandt was awarded a Nobel prize for the discovery.
One side note, a really bizarre fact of scientific progress is just how many discoveries have been made independently in the same year. I don’t know how or why it happens, maybe it’s likely to happen if certain scientific questions become a prevailing interest during a period of time, or maybe certain prior advancements in contemporary science make these independent discoveries inevitable, or maybe it’s something else, but either way you look at it, it’s really interesting. Some examples include the independent discovery of natural selection by both Darwin and Alfred Russel Wallace in 1858, the independent publication of periodic tables by both Dmitri Mendeleev and Julius Lothar Meyer in 1869, the discovery of the electron by J.J Thomas and Emil Wiechert in 1897, and Charles Scott Sherrington and Camillo Golgi both discovering the synapse in 1897.
The synthesis and administration of sex steroids
The first time a sex steroid was made artificially in a lab was in 1934 by Croatian-Swiss scientist Leopold Ruzicka, who successfully synthesized the male sex steroid androsterone. The next year, in 1935, he successfully synthesized testosterone, and in 1939 won the Nobel Prize in Chemistry for this and other achievements. From castrating male cattle in 8000 bce to synthesizing testosterone in a lab 10,000 years later isn’t too shabby if you ask me, we’re slow but you can do anything if you keep on grinding.
Not long after Ruzicka’s discoveries, the first male sex steroid product, a synthetic androgen called testosterone propionate was manufactured, marketed, and sold in 1937 under the brand-name Testoviron by Schering AG, a German pharmaceutical firm. The androgen was used to treat low testosterone in men and was the dominant form of testosterone used in treatment for nearly 25 years until longer-acting forms of synthetic testosterone were developed.
A few years prior in 1933, Schering AG developed and marketed the first female sex steroid product and the first commercially available sex steroid in history, a form of estrone called Emminen, which was isolated from the urine of pregnant women and used to treat symptoms of menopause, which is kind of weird, and later things do unfortunately get a bit weirder.
In the 1940s, a product called Premarin, a name which comes from the phrase, “pregnant mares’ urine,” was derived from the urine of pregnant mares by the pharmaceutical firm Wyeth, which is now owned by Pfizer. From the 1940s up till 2002 Premarin was one of the best selling pharmaceuticals in American history, and was used mostly to treat symptoms of menopause like hot flashes and vaginal dryness.
The understanding of hormones during this period was pretty simple and similar to ours today; it became common knowledge that estrogens decline after women’s fertility window closes, so the idea was that filling postmenopausal women up with estrogens again should fix their problems. Hormone replacement therapy (HRT) was seen as an indispensable part of women’s healthcare.
The Women’s Health Initiative study
In 1991 the National Institute of Health (NIH) began a study called the Women’s Health Initiative which was a longitudinal, randomized control trial (with a sample size of nearly 200,000 people) aiming to parse the safety and efficacy of Premarin in postmenopausal women. The NIH ended the trial earlier than planned for the sake of the safety of the participants, and the results of the study released in 2002 showed increased risk of pulmonary embolism, strokes, breast cancer, heart disease, and more as a consequence of exogenous female hormone administration. The results of the study were widely publicized and the sale of Premarin cratered. Lawsuits were filed en masse against Wyeth and Pfizer, who have since paid hundreds of millions of dollars to women who experienced significant adverse events after treatment.
A contradiction?
Why would hormones occurring naturally in women harm them when given exogenously? In the study, the randomized control trial was split into two groups, one non-hysterectomized group given a daily dose of estrogens derived from Premarin along with a synthetic form of progesterone, and one hysterectomized group given a daily dose of Premarin alone. My first guess is that a daily dose of estrogens simply fails to model the tightly-regulated way estrogens are released in healthy, premenopausal women, who normally have extremely wide-ranging estrogen levels even during a 24 hour period, not to mention over the course of the full menstrual cycle, with estrogen levels plummeting after the luteal phase.
As we’ve seen, the release of estrogens in healthy women is controlled by extremely sensitive negative feedback mechanisms, meaning when sufficient levels of estrogens are present in the blood, the hypothalamic-pituitary-gonadal axis (or HPG, the hormonal axis which stimulates estrogen production) shuts off to maintain a fine balance of the sex steroids in the body. Daily doses of estrogens just skirt over the complexity and delicate balance of this system, and so asking women to take them constantly just sounds crazy on the face of it.
Instead of getting selective pro-inflammatory or selective immune-dampening effects from estrogens when necessary, daily dosing might do a little bit of both when unnecessary. Enhanced immune responses from excess estrogens can cause coronary heart disease and clot formation, and attenuated immune function leads to things like cancer cell proliferation, for examples. The exact mechanisms of action need further study to conclude how exactly exogenous estrogens make things go wrong, but it’s important to understand that so far they seem to do so.
My second guess is that the form of estrogen active in Premarin, which is mostly estrone, might just have a much worse side-effect profile than other estrogens. Maybe if the postmenopausal women were given estradiol things might have gone better, so next we’ll see whether that’s the case by looking at the safety of other common hormonal treatments, like birth control.
Birth control and other popular hormone products
One shocking statistic that I never knew prior to researching this topic was that 90% of women ages 16 to 64 have used some form of birth control at some point in their lifetimes, and about 13% of reproductive-age women currently use hormonal contraceptives according to the CDC.
The first combined oral contraceptive (a contraceptive with more than one active steroid component) was called Enovid, a synthetic estradiol prodrug (which is a kind of precursor to another, desired substance) manufactured by G.D. Searle and Company, a pharmaceutical firm now owned by Pfizer, and approved for contraceptive use by the FDA in 1960. The drug was invented as a result of research conducted in the 1950s by Gregory Pincus, a Jewish-American biologist, John Rock, an American obstetrician, and Min Chueh Chang, a Chinese-American biologist whose work with his mentioned peers helped advance the science of in vitro fertilization. The team’s efforts were funded by Katharine McCormick, the second woman to graduate from MIT with a biology degree.
(It’s interesting to note that the emergence of the pill and the American sexual revolution both happened at around the same time, and you wouldn’t be wrong to guess that the former might be responsible for the latter. The involvement of Planned Parenthood’s Margaret Sanger in the development of The Pill is an understated element of its history and the history of the American sexual revolution. Before the influx of contraceptive research funding from Katharine McCormick, the first instance of funding came to the three inventors of The Pill from Margaret Sanger’s Planned Parenthood Federation of America, following a dinner between the scientists and Sanger. Sanger’s book Woman and The New Race, written in 1920, was one of the earliest works to advocate for a total reinvention of sexual attitudes, marriage, and society as a whole, and illustrates the perspectives underpinning the pill’s creation).
Different forms of the pill were manufactured by different brands with different amounts of active hormonal ingredients, but the common thread between them was the central importance of estradiol in the contraceptives most widely sold. The pill was effective, reducing unwanted pregnancies by around 80%. But was the pill safe? Let’s take a look at some of the same adverse events seen in the Women’s Health Initiative study, like rates of cancer, stroke, and heart disease, and see if recent meta-analyses shed some light.
Breast cancer and birth control
In a good meta-study of the connection between birth control and breast cancer risk, which reviewed the findings of forty-two papers from 2009-2020 involving nearly 120,000 women combined, it was found that birth control markedly increased the risk of developing breast cancer:
“To perform a meta-analysis of case-control studies that addressed the association between oral contraceptive pills (OC) use and breast cancer (BrCa), PubMED (MEDLINE), Embase, and the Cochrane Library were searched to identify case-control studies of OC and BrCa published between 2009 and 2020. We used the DerSimonian–Laird method to compute pooled odds ratios (ORs) and confidence intervals (CIs), and the Mantel–Haenszel test to assess the association between OC use and cancer. Forty-two studies were identified that met the inclusion criteria and we included a total of 110,580 women (30,778 into the BrCa group and 79,802 into the control group, of which 15,722 and 38,334 were using OC, respectively). The conducted meta-analysis showed that the use of OC was associated with a significantly increased risk of BrCa in general,”
Let’s take a look at some other diseases like heart disease to see what we find.
Heart disease and birth control
In a huge meta-study that looked at whether or not birth control causes heart attacks and stroke, the researchers found that birth control increases the risk of myocardial infarction (a blockage in the heart) and ischemic stroke (a blockage in the brain) by almost two times. The study looked at whether risk varied by type of birth control, and the risk seemed pretty much even across most types besides ones comprised of more estradiol, which were a bit more risky:
“In total, we identified 1298 publications through the search strategy. We included 28 publications reporting on 24 studies. COC users were at increased risk of myocardial infarction or ischemic stroke compared with non‐users: relative risk (RR) 1.6 (95% CI 1.3‐1.9).These RRs were similar for myocardial infarction (1.6, 95% CI 1.2 to 2.1) and ischemic stroke (1.7, 95% CI 1.5 to 1.9). The risks did not vary clearly according to the generation of progestagen or according to progestagen type. When we stratified preparations according to estrogen dose, the risk of myocardial infarction or ischemic stroke seemed to increase with higher doses of estrogen.”
What about the effects of birth control on mental health, which the Women’s Health Initiative study didn’t look at? Do women on birth control have better or worse mental health outcomes?
Depression and birth control
The research landscape on whether or not hormonal contraceptives improve or worsen women’s mental health is tenuous at best. A few prominent meta-analyses have found no association between birth control and depression, and some even found positive associations. But on the other hand, a massive, longitudinal study in Denmark which followed nearly half a million women for over 8 years found that contraceptive use doubled the risk of suicide attempts, and tripled the risk of successful suicide. And another enormous study from Denmark with over one million women, which followed up with each study participant a bit over 6 years after first contraceptive use, found a nontrivial increase in the risk of developing depression.
What’s the deal?
One study in the Netherlands tried to resolve these discrepancies by pointing to some pretty simple solutions: most studies which find no or even positive effects from birth control are usually shorter term, and are also retrospective, which is what I’ve seen so far with most hormonal studies in general. Retrospective studies take the medical records of large numbers of women, control as best they can for factors like age, other confounds, and contraceptive use, then look at whether or not women segmented by contraceptive use developed diseases like breast cancer more often than their non-birth control taking counterparts over an arbitrary period of years. Retrospective studies can be a very powerful tool, but are commonly plagued by unaccounted confounds and biases, so prospective studies, which do the opposite of retrospective analyses and closely observe live cohorts over the course of a research period, offer a lot more precision.
The Netherlands study was a prospective analysis following a large group of young Dutch women, and found a “small but robust increased risk for experiencing an episode of major depressive disorder, especially among women with no history of major depressive disorder in adolescence. Understanding the potential side effects of oral contraceptives.” The longer term studies and the prospective ones as well seem to consistently find a link between contraceptive use and depression. More research needs to be done here, but the effect of contraceptives on mental health so far don’t seem great.
My two cents
Altogether the trend of widespread birth control prescription seems like one of the biggest instances of medical malpractice in history. Women are encouraged to take on significant risk to mind and body for very little gain, if any at all.
What about synthetic androgen treatments?
So far it seems that synthetic estrogens pose significant risks to physical and mental well-being, but what about things like anabolic steroids?
In the 1954 international weightlifting championships held in Vienna, the Soviet Union stunned the world by winning gold in more events than any competing nation. It was widely speculated that the Soviet Union was giving shots of testosterone to their athletes to gain a marked edge, and John Ziegler, a scientist and doctor for the American weightlifting team, partnered with Ciba pharmaceuticals to develop a performance-enhancer with a longer half-life than exogenous testosterone. This led to the development of methandrostenolone in 1958, a synthetic testosterone.
In the 1960s use of methandrostenolone became widespread in professional sports, and other synthetic steroids were being developed and marketed at the same time like Oxymetholone and Stanozolol, but proscriptions against steroid-use in professional US sports didn’t come till decades later. Anabolic steroids were classified as controlled substances only in 1990 after the passage of the Anabolic Steroids Control Act, following growing concern over their health risks. And today we know that long term use of anabolic steroids causes things like hypogonadotropic hypogonadism, which causes testicular decay in men and failure to express estrogens in women, and also severe damage to liver function, blunted insulin sensitivity, and a host of strange psychological issues like increased interest in opioids, increased proclivity to violence, increased incidence of psychosis and paranoia, increased incidence of suicide, with one study finding suicide and homicide to be the primary cause of death among steroid users, and more.
To gain a more human-level perspective on the subject I read some accounts of pro athletes who admitted using steroids, and the most wild account was probably from MLB’s six-time all-star Jose Canseco, who blew the whistle on widespread steroid use in Major League Baseball in his book Juiced. In recent interviews Canseco describes being sterile from his use of steroids, with his body being unable to produce its own testosterone. His life story is just completely off the rails and can only be explained by regular use of some kind of hard drug, but it turns out the hard drug was just anabolic steroids, which can apparently have similar effects on the brain as amphetamines. Canseco experienced psychotic periods of violence, frequent arrests for domestic abuse, and frequent bouts of saying crazy things online, all caused by taking a synthetic male hormone. He’s become a big advocate against “juicing” and I understand why—steroids are nuts.
Brief overview
To recap—Exogenous sex hormones in general seem relatively risky even when they’re used in same-sex contexts, which agrees with common sense considering how complicated and sensitive the human hormonal system is, as we’ve seen. The hypothalamic-pituitary-gonadal axis, the growth hormone/insulin-like growth factor-1 axis, and everything else associated with hormones present a constellation of feedback loops and genetic and non-genetic pathways purposed on fostering growth and maintaining the crimson, pulsating, vital scales of health.
Hormones are less like keys to unlock desired physiological doors and more like bits of relevant information in a stock exchange, like a relative strength index or moving average, both of which are signals that give some idea of historical and potential growth of an asset. These signals are powerful, but they only go so far to explain or predict the hidden universe of individual and corporate decisions behind real-time and future market movements, they provide limited insight into a system of much wider context. We understand that hormones mean growth and development, but they also mean and are part of something so much more, they’re a note in Schubert’s piano trio in E-flat major, the note can’t give you the symphony.
We’re going to look at cross-sex hormone administration next and, knowing what we know about hormones in general and the way they affect healthy people when given exogenously, it’s appropriate now to have some skepticism about the claim that cross-sex hormones are essential for anyone’s health. We’ve established a firm foundation with the best research available, and the best research shows that hormones are not something to casually mess around with. Any conclusions showing the contrary should be met with eyebrows raised so high they greet God in heaven.
But we’ll look at the best data we can find before drawing our conclusions.
Cross-sex hormones and mental health
The first thing to mention about the information geography around cross-sex hormone treatment is that there is a dearth of quality research. Most studies in the domain have almost no methodological rigor and don’t control for things like substance use before or after cross-sex hormone treatment, psychiatric history of patients, psychiatric treatment started before or after cross-sex hormone treatment, time-passed since initiation of cross-sex hormone treatment, and much more, and most sample sizes are impressively small. This review of the literature concerning the subject of cross-sex treatments and suicide and suicidal ideation, for example, does a good job describing the lack of necessary controls across the research landscape, with dozens and dozens of papers having no meaningful controls at all.
At this time, without establishing a firm background understanding of hormones as we’ve done and instead taking the current cross-sex research at face value, we couldn’t confidently say whether or not cross-sex hormone therapy has positive or negative influence on mental health, since the studies are so poor. Several European nations have recently restricted the use of “gender affirming” cross-sex treatments in adolescents due to this extreme paucity of usable research in the domain.
The Dutch Protocol and gender-distressed minors
Previously the research used to justify cross-sex hormone treatments in gender-dysphoric minors was a small longitudinal study in the Netherlands which found positive treatment outcomes, and these outcomes were used to establish the “Dutch Protocol,” or the affirmative, hormone-supplying approach to gender dysphoria which quickly became an international standard. But in recent years the shaky foundation of the study has come to light, with a recent attempt to replicate its findings in the U.K. failing entirely. Replication of research is a scientific gold standard, but seeing the methodological design of the Dutch study, it’s no wonder why replication failed:
“…the Dutch study’s lead author, Annelou de Vries, has admitted that “resolution of gender dysphoria” was its “main finding.” But this finding was based on a highly questionable use of the Utrecht Gender Dysphoria Scale—a measure originally developed for diagnostic purposes, not to assess treatment outcomes. The scale is sex-specific, which means that biological males and biological females are given different versions of it. Among other differences, the female version includes questions on menstruation while the male version includes questions about erections. In their follow-up assessments, the Dutch team gave boys who had undergone hormonal treatments the girls’ scale and girls who had undergone hormonal treatments the boys’ scale. Thus, biological males were asked whether experiencing menstruation caused them distress. Since even boys who “transition” do not get periods, those who answered the questionnaire reported a low level of distress. In other words, the plummeting scores in gender dysphoria that the Dutch team reported as their “main finding” was not necessarily due to actually resolved dysphoria, but more likely to switching the scales.”
One interesting element of the Dutch protocol I don’t see mentioned often is that the research was funded by Ferring Pharmaceuticals, the manufacturer of the puberty-suppressing hormone treatment Triptorelin, which seems like a pretty big conflict of interest. I would not trust a pharmaceutical retailer to conduct honest research on whether or not its drugs are safe and helpful, but maybe that’s just me.
At any rate, the best trans medicine research in the world having unintelligible design, being funded by a pharmaceutical company, and immediately failing replication illustrates the state of things in the domain, but isn’t inconsistent with the history of hormones in general, as we’ve seen. The history of hormone treatments is the history of throwing caution to the wind, mostly to the benefit of the powerful. More investigation is necessary to ensure the safety of those seeking relief from legitimate mental challenges, the current approach is ineffective at best, and at worst potentially harmful.
Cross-sex hormones and cardiovascular disease
The best studies I’ve found on cross-sex treatments concern more easily quantifiable causes of mortality than mental health status, like cardiovascular disease risk, with most studies here finding significantly increased risk of certain cardiovascular events in men being administered feminizing hormones, and with some studies finding cardiovascular disease to be a leading cause of death in post-treatment men.
One retrospective study from a few years ago with decent controls (though there were still some serious limitations like accounting for comorbidities), large sample, and an extremely large comparison group found little difference in rates of myocardial infarctions between men taking estrogens and untreated comparison groups, but found significant difference in the rates of venous thromboembolism (or VTE, clots present in the lower body, heart, or lungs) in estrogen exposed men relative to non-estrogen exposed men and women. In men treated with estrogens, the risk of clot formation increased significantly following the first two years after treatment initiation, and the risk of stroke increased significantly as well, but only after the first six years of treatment, which is interesting:
“Results further indicate that the increases in VTE and ischemic stroke rates were most pronounced among transfeminine participants who initiated estrogen therapy during follow-up and that the patterns of these increases differed substantially from those reported in previous research. For example, in a clinical trial of hormone replacement therapy in postmenopausal women, VTE rates increased relatively rapidly after the intervention began and seemed to decline and then plateau by 5 years of follow-up (18). Likewise, in a case-control study of VTE and estradiol use in Sweden, the risk was elevated only during the first year after the start of therapy (19). In contrast, in our estrogen initiation cohort, VTE rates increased only after 2 years of follow-up and continued to rise for another 5 to 6 years. Likewise, the ischemic stroke rates in the estrogen initiation and 2 reference cohorts did not differ during the first 6 years of follow-up but clearly diverged afterward.”
The study references another large experiment conducted in Amsterdam in the 90s which found similar results:
“One of the largest studies to date included a cohort of 816 transfeminine participants who received oral ethinyl estradiol (100 mcg/d) or transdermal estradiol and 293 transmasculine patients who received parenteral testosterone esters or oral testosterone undecanoate. The study participants were seen in the outpatient department of Free University Hospital in Amsterdam between 1975 and 1994. Among the transfeminine participants, 45 cases of VTE occurred, only 5 of which arose after surgery. With the general male population of the Netherlands used as a reference, this number of VTE cases was 20-fold higher than expected.”
The same study found that cross-sex testosterone treatments in women showed little effect on cardiovascular disease risk, and most other studies I’ve seen on testosterone administration in female pulmonary health seems to show little if any influence. But exogenous testosterone causes a litany of other problems from acne to PCOS to premature baldness, and likely causes the other psychotic symptoms we’ve seen follow from androgen administration in men.
Cross-sex hormones and fertility
It’s been noted that cross-sex treatments effectively impair the normal reproductive function of respective patients, and whether these impairments are reversible in treated adults is currently unknown. Treatments that suppress puberty in adolescents most likely permanently alter reproductive capacity later in life considering what we know about hormones and growth, but research here has yet to proceed at a pace or scale necessary to clarify unanswered questions.
Here’s a study done on these matters of fertility finding that cross-sex hormone patients become infertile, but the findings should be approached with caution:
“Transgender individuals who undergo gender-affirming medical or surgical therapies are at risk for infertility. Suppression of puberty with gonadotropin-releasing hormone agonist analogs (GnRHa) in the pediatric transgender patient can pause the maturation of germ cells, and thus, affect fertility potential. Testosterone therapy in transgender men can suppress ovulation and alter ovarian histology, while estrogen therapy in transgender women can lead to impaired spermatogenesis and testicular atrophy. The effect of hormone therapy on fertility is potentially reversible, but the extent is unclear.”
Cross-sex hormones in perspective
The use of cross-sex hormones to treat gender-dysphoria in minors and in adults will likely be a significant entry into the books of medical malpractice in the future.
Insufficient research is being conducted on the safety and effectiveness of cross-sex hormone treatments, possibly out of the self-interest of pharmaceutical entities, ideological reasons, or even well-meaning but uninformed assumptions about the efficacy of these medicines. Whether this state of affairs will change is unlikely, but the hope of this article is to present a clear and broad picture to supplement the gaps in our understanding and come to a reasonable view.
So far we’ve seen hormones in context, the way they operate in the body naturally and the way they operate when given externally. We have sufficient reason to believe that almost all hormone treatments should be treated with caution. And we currently have evidence to be wary of cross-sex treatments considering the paucity of quality investigations, the recent failure to replicate previous positive findings, and the dangerous outcomes shown by what little decent research we have.
Cross-sex hormone treatments present a highly contentious cultural and political issue, but the health and well-being of real people with real struggles and real desire to ameliorate feelings of distress should be viewed without the muddying influence of partisan politics, and only with the dispassionate rigor of science. An honest look into the matter shows that calls to pause gender affirming care are both justified and compassionate, considering that lives are at stake.
Hopefully this overview has been helpful to those on both sides of this political fight, and hopefully the political fights of the future can be resolved with the similar broad investigations.







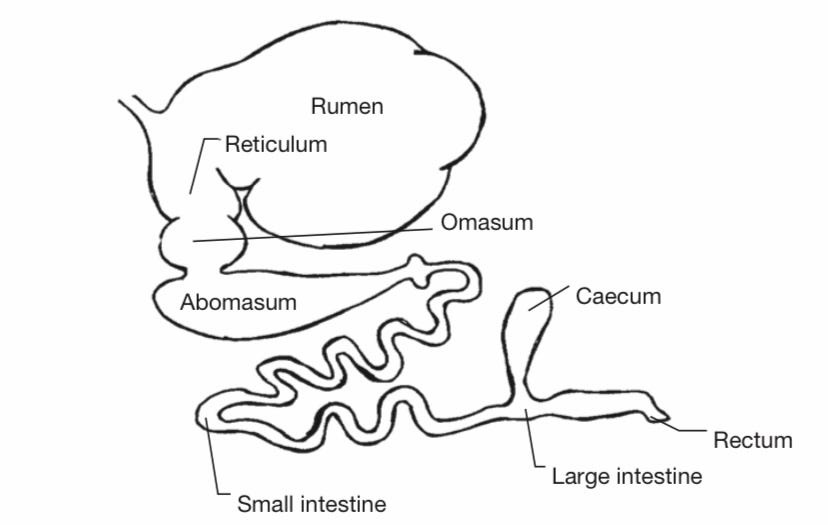
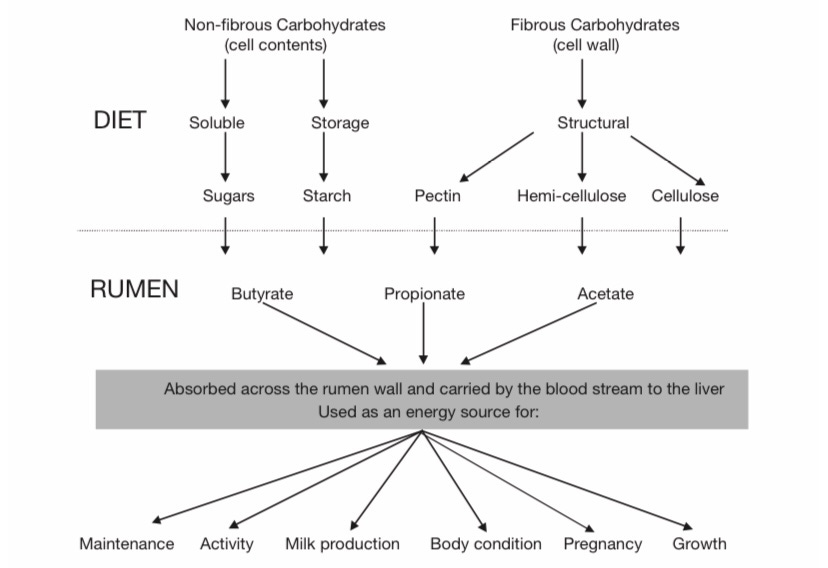





"We’ve established a firm foundation with the best research available, and the best research shows that hormones are not something to casually mess around with. Any conclusions showing the contrary should be met with eyebrows raised so high they greet God in heaven. "
That seems overly generic as a conclusion.
At the very least, it should be necessary to amend this conclusion by studying on insulin-based treatments for diabetics and thyoxin-based treatments for hypothyroisdism.
This should not be cause to reject the conclusion of this essay outright. The most important facet of current sex-based treatments is the dearth of good studies validating such treatments. It should, however, be cause to nuance one's opinion on hormone-based treatments.
One area that was not touched on here - and seems like potentially a more judicious use of hormones - is hormone replacement therapy for older people whose natural levels decline